Case Studies: Research & development
Patient Advisory Board for a Clinical Trial
Project brief
Dr. Lode Dewulf has relevant experience as patient, carer, practising physician and also industry executive. He has been among the thought leaders of patient-industry collaboration since several years, and contributed to this study case as co-founder and managing partner of Corvalus. Lode is currently working as Chief Patient Officer at Servier Group and previously worked as Chief Medical Affairs Officer and then Chief Patient Affairs Officer at UCB.
Dr. Andreas Reimann is Co-Founder and Managing Partner of admedicum® Business for Patients. Besides a lot more patient-industry experience he has been actively working with industry as the CEO of the German Cystic Fibrosis Foundation for more than 12 years.
Situation
A midsize pharma company is about to start a clinical trial in children aged 6-12 years, suffering from Cystic Fibrosis (CF). The clinical trial is prepared, and the investigator kick-off meeting scheduled. A Scientific Advisory Board with renowned Key Opinion Leaders (KOLs) across Europe has been established.
An International Project Team is to supervise the clinical trial. The team members have an excellent scientific and medical understanding of the disease. Some of the team members have individual contacts with several CF patient organizations (PO) across Europe.
Objective
The pharma company project team wants to set up a Patient Advisory Board for review of the clinical trial during execution. With the support of the Patient Advisory Board, the project team hopes to enhance patient recruitment and retention during the trial.
Challenge
Effectively setting up a Patient Advisory Board, managing internal and external expectations.
Procedure
Approach
- The project team has identified several parents from different national POs who would be willing to join the Patient Advisory Board. Although the head of clinical research supports the project, he did not grant additional resources nor budget.
- The project team asks their compliance officer’s help to set up a charter and honoraria for the Patient Advisory Board. However, the compliance officer questions the value of a Patient Advisory Board altogether. He states that the goals for the Patient Advisory Board are not clear to him and would think that parents of patients would lack the required expertise. In addition, he is concerned that involving parents in a concrete development program could be perceived as influencing patients with the objective to support future commercialization.
- After intense internal discussions, the project team manages to get support from the compliance department for a suitable approach to reach out to selected parents on an individual basis. During the preparation phase for the first Patient Advisory Board meeting, several difficulties arise:
- 8 parents from 5 different countries agree to attend the first meeting. 4 of the 8 parents’ English is not sufficient and translation services are required. This is not budgeted.
- While preparing the Patient Advisory Board Meeting agenda, it turns out that the level of knowledge of the disease and about drug development of the envisaged participants is very heterogeneous, challenging the impact of the meeting.
- 5 parents request reimbursement for the cost involved with the care of their children during their trip. This is not budgeted.
- Parents complain about slow communication and unclear agenda / expectations.
- Among parents the only date mutually agreeable is a Saturday, which in turn is difficult for the project team members, who are already stretched to their limits.
- Several parents do not agree to sign the confidentiality agreement which the legal department insists upon.
Evaluation
What went well
As with KOLs, building personal, trustful contacts with patient/parent experts is very helpful. Establishing these relationships requires time, and, in recognition of that, the team started working on this long before the clinical development started. Also, the team identified the opportunity to work with parents directly to enhance recruitment and retention.
What can be improved?
The team also needs to challenge their approach to setting up a Patient Advisory Board with regards to:
- Timing: involvement of patients/parents prior to key decisions on the trial can add more value and makes it easier to explain the benefits of patient engagement to key stakeholders, both internally (e.g. to the compliance officer) and externally (e.g. to the patient experts you want to win over for collaboration).
- Preparation: the team had difficulties to “sell” the value of the Patient Advisory Board to the head of clinical research (who did not grant additional budget nor resources) and the compliance officer (who did not see any value at first). To prevent this from happening, it is important to have clarity on the why, what and how (see case study "First Time Contact to Patients from the Industry's View" and checklist for preparing a collaboration by EUPATI, ABPI and National Voices). Collaboration with patients regularly requires more time and attention than KOL liaison management. Lack of capacity and budget can easily hamper a successful collaboration later in the process.
- Performance: timely internal preparation and clarity would have had a positive impact on the selection of the patient/parent experts and the efficiency of the process.
Considerations and recommendations
Preparing for engagement of patients in clinical development
Consider involving patients or parent experts early on in the clinical development process, way before the clinical trial design is determined. Their expertise and input can be very valuable in the conceptual stages. The following tools give guidance on who to involve, what to consider and how to set up an efficient Patient Advisory Board. They cover aspects like adequate documentation before, during and after the actual meetings, the number and nature of participants (from both sides), the language challenge and compensation at fair market value:
- Tool "Considerations for Implementing a Patient Expert Group" (DIA)
- Tool "Building a Patient Advisory Board"
EUPATI provides an interesting case study on patient involvement by setting up an Advisory Board on a Clinical Trial Design.
Preparing a Patient Advisory Board
Before you start selecting patients as candidates for a Patient Advisory Board, have a plan for the why, what and how. Ask yourself key questions about the goals of the interaction and who you'll need to achieve these goals. A list of proposed questions can be helpful in the development of a Patient Advisory Board. This exercise can result in a detailed draft charter for the expert group, which could be of great value for getting internal support, but even more so for the communication with your patient expert candidates. Don’t miss the opportunity to give internal stakeholders and patient experts a chance to comment and “co-create” such a charter! Your colleagues from legal, compliance and other support functions and of course your patient partners will much appreciate it!
Several publicly available sources exist for checklists that can be used in preparing for Patient Advisory Boards.
For planning and conducting a Patient Advisory Board in a structured and detailed manner, we especially recommend following sections of the DIA Considerations Guide to Implementing Patient-Centric Initiatives in Health Care Product Development:
- Considerations for preparing your organization to engage expert patients or patient groups (page 16)
- Communication plan (page 18)
- Patient retention and compliance (page 30)
- Measuring success and capturing learnings (page 33)
Of great value are the tools and checklists of the Annual Report 2016 of the Clinical Trials Transformation Initiative (CTTI). Here, we would like to recommend the very hands-on checklist on Assessment of Patient Group Internal Aspects (Excel file).
Selection of patient experts
Even though you may have good personal contacts with certain patient experts, they may not necessarily be the best choice for the purpose of your Patient Advisory Board. You should carefully select the right people, subject to the kind of skills and expertise required. It is in the best interest of patients that you work with the most skilled patient experts. Some tips and tricks on finding and selecting the right patients experts:
- Tool "Patient identification” EUPATI (how to identify the right patient expert)
- Ask your medical advisors / KOLs for their contacts to patient organizations and patient experts, internationally, and arrange personal introductions.
- Start with personal conversations before moving to group events to further build the relationship, understand the level of expertise and the expectations of the respective person.
Help us improve this guide
Submit Feedback
Tool
Considerations for Implementing Expert Patient or Patient Group Input
Recommended contributors :
- Program leaders
- Patient liaisons
- Sponsor representatives
- Clinical investigators
- Research team
- Trial site staff
- IRB
- Expert patient(s)/patient group representatives
Communicating with patients throughout the program
- How does the phase of drug/biologic/device development process covered by this program impact communication with patients?
- What translation and/or cultural adaptions are necessary?
- Wat language will be used to communicate with and about the patients?
- Are research questions and procedures culturally sensitive and appropriate?
- How will patients be referred to (e.g. “subject” vs. “patient” vs. “participant”)?
- What is the communication plan for patients throughout the program?
- Message content
- Audience
- Messenger
- Delivery mechanisms
- Timing
- Feedback mechanisms
- What feedback mechanisms and processes are in place for the patients to comment on sites, investigators, and the study participant experience?
- What role will social media play in the communications?
- How is social media defined?
- How can social media be utilized (e.g. for trial recruitment, to educate patients)?
- What restrictions should there be, if any? Why?
- How will those limits be communicated and enforced?
- What methods will be used to interact with patients and other stakeholders?
- Focus groups
- Interviews
- Surveys
- Inclusion in advisory councils
- Inclusion in meetings with researchers
- What data/information can and will be shared with the patients and when?
- Aggregate (de-identified)
- Patient-specific
- What are the restrictions (propieratry and regulatory) constraining the release of data?
- How do we ensure that this information is shared in patient-friendly language? How will that be determined/monitored?
Additional resources
Communication Handbook for Clinical Trials.
Guidance for Biomedical HIV Prevention Trials, p 37-38: “Stakeholder education plan.”
Download
Checklist
Preparing a collaboration
Defining the interaction
Patients, patient representatives and industry should take responsibility to ensure interactions are meaningful by clearly defined processes and actions, progressed to timelines. In addition, all participants should be prepared for the interaction.
Prior to each interaction, agree mutually on (where applicable):
- The objective of project involving patients and/or areas of common interest to establish agreed structured interaction, providing all parties with necessary protection with regards to independence, privacy, confidentiality and expectations (see section 11. written agreement)
- The type of input and mandate of the involved person
- The tools and methods of interaction, e.g. types and frequency of meetings, ground rules, conflict resolution, evaluation
- Desired patient / patient partner organisation to foster long-term working partnerships, with independence ensured (in scope)
- The profile of the type of patient/s or patient representatives/s to be involved and their number
- How activity outputs will be used and ownership of outputs
- How and when the patient/s involved will be informed of outcomes
- Contractual terms and conditions including consent and compensation (see section 11, written agreement).
- Other elements according to the specific project
Source
Checklist
Preparing a collaboration
The four key principles for collaboration:
1. Clarity of Purpose
Each party should be clear about the reason for and the planned outcome of the collaboration – and the ultimate benefit for patients
2. Integrity
Each party should act and be seen to act honestly and with integrity at all times
3. Independence
Each party should maintain their independence
4. Transparency
Each party should be open and honest about the purpose of the collaboration and be able to account publicly for the associated activities and any exchanges of funding
Using this guide: a checklist
- Has there been a frank discussion about the purpose and expected benefits of the collaboration, and any risks, addressing all the issues in this guide?
- Are the objectives and planned outcomes of the collaboration specified?
- Are the roles of each partner and reporting mechanisms specified?
- Has a written agreement or contract been put in place, which sets out how each party will adhere to the four key principles?
- Is there a named senior individual accountable for managing and maintaining the relationship and monitoring adherence to the four key principles?
- Is information about the collaboration published on the company and charity websites?
- Can each party confidently explain the collaboration in public?
Source: National Voices, The Association of the British Pharmaceutical Industry (ABPI) (2015): Working
Download
Checklist
Patient identification/interaction
Patient identification/interaction
There are many ways to identify patients to be involved in an interaction. The main routs are through:
- existing patient organisations
- EUPATI or similar project
- advertising opportunities for patient participation
- existing relationships with healthcare providers, hospitals and researchers and other agencies
- unsolicited requests previously made by interested parties
- existing advisory boards / groups (e.g. EFPIA Think Tank, Patients and Consumers Working Party at the EMA)
- this party agencies
Source: European Patients’Academy on Therapeutic Innovation (EUPATI) (2016): Guidance for patient involvement for industry-led medicines R&D. (12/06/17)
Tool
List of useful conferences
Conferences with and for patients
Rare diseases conferences with and for patients
The Global Orphand Drug Conference and Expo
Indication specific conferences with and for patients
Tool
Defining role patients
Although you may not have selected the expert patients or patient groups (EP/PGs) yet, outlining their roles and responsibilities at this stage helps to define your needs. Keep in mind that EP/PG roles may vary at different of the program or may evolve in response to new requirements. Once selected, discuss the roles with your EP/PGs to clarify what they can contribute based on their unique expertise and experience and avoid misunderstandings at the outset, e.g., if they’re expecting to have a partnership role but you’ve designed reactor role (see Types of Patient Roles chart below).
| Patient Role | Examples | Engagement Level |
| Partnership role |
| High |
| Advisor role |
| Moderate |
| Reactor role |
| Low |
| Trial or study participant |
| None |
Source: DIA (2017): Considerations Guide to Implementing Patient-Centric Initiatives in Health Care Product
Development. (02/06/17)
Contact admedicum
Get in Touch
TOOL
Considerations for Implementing Expert Patient/Patient Group Input
DIA's Considerations Guide to Implementing Patient-Centric Initiatives in Health Care Product Development
Recommended Contributors:
- Program leader
- Patient liaisons
- Sponsor representatives
- Clinical investigators
- Research team
- Trial site staff
- IRB
- Expert patient(s)/Patient Group representatives
Communicating with Patients throughout the Program
- How does the phase of drug/biologic/device development process covered by this program impact communication with patients?
- What translation and/or cultural adaptations will be needed? How can the Expert Patient/Patient Group help identify and prepare for those adaptations?
- What language will be used to communicate with and about the patients?
- Are research questions and procedures culturally sensitive and appropriate?
- How will patients be referred to (e.g., “subject” vs. “patient” vs. “participant”)?
- What is the communication plan for patients throughout the program?
- Message content
- Audience
- Messenger
- Delivery mechanisms
- Timing
- Feedback mechanisms
- What feedback mechanisms and processes are in place for the patients to comment on sites, investigators, and the study participant experience?
- What role will social media play in the communications?
- How is social media defined?
- What restrictions should there be, if any?
- How can social media be used to advantage (e.g., for trial recruitment, to educate patients)?
- What limits should be placed on use of social media, if any? Why?
- How will those limits be communicated and enforced?
- What methods will be used to interact with patients and other stakeholders?
- Focus groups
- Interviews
- Surveys
- Inclusion on advisory councils
- Inclusion in meetings with researchers
- What data/information can and will be shared with the patients and when?
- Aggregate (de-identified)
- Patient-specific
- What are the restrictions (proprietary and regulatory) constraining the release of data?
- How do we ensure that this information is shared in patient-friendly language? How will that be determined/monitored?
Source: DIA Considerations Guide 2017): http://engage.diaglobal.org/PatientEngagementConsiderationsGuide.html
TOOL
Building an Advisory Board
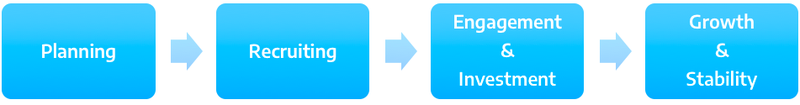
Planning
- Identify need for Patient Advisory Board
- Clarify goals of Patient Advisory Board
- Identify staff in charge of the Board
- Create vision, mission, statement
- Make logistic arrangements for Board meeting
- How long?
- How often?
- How many people?
- What incentives can be provided?
- How to proceed with travel cost reimbursement?
Recruitment
- Roll out recruitment plan
- Outreach to potential advisors
- Spread the word internally about the role of Patient Advisory Board and its importance
Engagement & Investment
- Orientation
- Goals
- Role
- Expectations
- Rules
- Establish relationship with new advisors
Growth & Sustainability
- Create a system to continue building relationships with patients
- Create a framework of regular meetings
- Maintain communication with patients, staff and leadership: develop a continuing communication system
- Explore opportunities for partnerships and growth
- Track and share successes
- Evaluate challenges and move towards solutions
Source: Modification of a toolkit for building an advisory board in hospital setting. Angel, L. (2014): Starting and sustaining a Patient Advisory Board. Patient Liaison Handbook. Family Health Center-San Francisco General Hospital. UCSF-Family Community Medicine. Source: https://www.pcpcc.org/resource/starting-and-sustaining-patient-advisory-board (02/06/17)
Download
TOOL
10 Questions for Building a Patient Advisory Board
This list of questions is non-exhaustive but may help with making a strong start. We recommend to discuss these questions with internal and external people you trust and may want to have on board.
- Why do you want to set up a Patient Advisory Board? Define a mission statement.
- What are the specific objectives and the anticipated outputs?
- Are you willing to share these objectives, critically discuss and openly communicate them to all prospective board members? (If not, this may be prohibitive for setting up such a board.)
- If confidentiality of content is required, how are you going to provide for it? E.g. will participants have to sign a Confidential Disclosure Agreement (CDA) prior to working with you? What does that mean to the process and the selection of candidates?
- How often do you think the Patient Advisory Board needs to meet and how much time should the patient advisor and your organization dedicate to the Patient Advisory Board? Is that realistic?
- Do you have your management’s support for working with the Patient Advisory Board and for spending the time, resources and money on such an effort?
- What are the skills/profiles of the participating patient experts you want as advisors?
- Who should participate in the Patient Advisory Board meeting(s)?
- Who will chair the Patient Advisory Board and who's in charge of the follow-up? Will you need an external facilitator?
- How will the logistics, compensation and legal compliance be provided for, including contractual arrangements? As you are working with patients, facilities may need to be adapted.
For more general guidance and detailed questions have a look at these sources:
EFPIA Guide “Working Together with Patient-Groups”
D.I.A. ConsiderationsGuide to Implementing Patient-Centric Initiatives
Download
Draft Charter for a Patient Advisory Board
This charter is designed for open communication with all potential Patient Advisory Board members inside and outside your organization and any other party interested in the purpose of the Patient Advisory Board and the nature of the attendees. Please note that Transparency Codes apply.
We strongly recommend to use this only as a proposal for clarifying your own thinking first and then ask all other involved stakeholders (patient experts, Key Opinion Leaders, others) to comment and add as appropriate. You may even start with an open discussion before drafting with some of the potential advisors you know you want to have on the board. The final charter should be the reflection of the major points agreed upon between the collaborators about why to collaborate on what and how to do that.
Purpose, mission and objectives
The “insert Name of the Board” shall contribute to (specify)…
- Describe what your company is currently doing, why this requires patient advice and what you hope to achieve from it.
- Describe the specific objectives the Board should strive to achieve in as much detail as possible (“to learn / get insight / discuss…”). Try to put yourself into the shoes of the potential Patient Advisory Board member reading this who will decide whether to join or not based on this charter.
- Input from (already) appointed members of the board, both inside and outside your organization, is valuable to improve the charter.
Members and responsibilities
The (insert Name of the Board) consists of (specify)…
Describe the desired profile of the external and internal board members and their expected roles:
- Who should chair the Board? Is an independent, external chair required? And what are this person’s responsibilities:
The Board will be chaired by (insert name and function). The chairperson is responsible for preparation of the meeting, including drafting the agenda and sending it to the Board for input, finalizing the agenda, moderation of the meeting itself, drafting the meeting minutes and follow-up.
- Describe the patient advisors on the board and their roles, names and backgrounds/functions:
There will be 4 patient advisors on the board:- a patient advocate on behalf of patient organization A
- a patient advocate on behalf of patient organization B
- 2 patients diagnosed with (specify indication), who have experience with (specify, e.g. specific treatment, a specific patient support program or more general “clinical development in disease X”)
- Describe the employees of your organization who should be on the board and their expected roles, names and functions:
There will be (number) company representatives on the board:- The head of clinical development
- The international project lead
- The head of market access
- etc.
- Describe any other external advisors you may want to have on your board and their expected roles:
- A physician
- An experienced patient nurse
- A social worker
- A caretaker
- etc.
Organization and logistics of the Patient Advisory Board
The “insert Name of the Board” will meet (specify)…
- Describe in detail the frequency of meetings, whether they are in person or via phone/web conference, the duration and location.
- Clarify whether confidentiality agreements are in place and provide for the board members’ freedom to communicate about the confidential information between each other:
The Board meetings will be subject to confidentiality as agreed individually in written form between the company and the board members.
- Make sure provisions are taken to ensure all Board members “speak the same language”, hereby taking into account not everyone may be fluent in English and avoiding misinterpretations. Also, local Boards can be in a different language:
All Board materials and the board meetings will be in English language. Where needed translation of materials and during meetings will be provided for.
- Make sure all participants agree on time needed for preparation:
Board members shall prepare for the board meetings based on the material provided by the Chair, which shall not exceed more than (…specify estimated time…) per board session.
- Provide for detailed loops before, during and after each session regarding possibly required adaptations.
Source: This charter has been developed by admedicum® Business for Patients GmbH
Download
Checklist
Patient identification/interaction
Patient identification/interaction
There are many ways to identify patients to be involved in an interaction. The main routs are through:
- existing patient organisations
- EUPATI or similar project
- advertising opportunities for patient participation
- existing relationships with healthcare providers, hospitals and researchers and other agencies
- unsolicited requests previously made by interested parties
- existing advisory boards / groups (e.g. EFPIA Think Tank, Patients and Consumers Working Party at the EMA)
- this party agencies
Source: European Patients’Academy on Therapeutic Innovation (EUPATI) (2016): Guidance for patient involvement for industry-led medicines R&D. (12/06/17)
Checklist
Preparing a collaboration
Defining the interaction
Patients, patient representatives and industry should take responsibility to ensure interactions are meaningful by clearly defined processes and actions, progressed to timelines. In addition, all participants should be prepared for the interaction.
Prior to each interaction, agree mutually on (where applicable):
- The objective of project involving patients and/or areas of common interest to establish agreed structured interaction, providing all parties with necessary protection with regards to independence, privacy, confidentiality and expectations (see section 11. written agreement)
- The type of input and mandate of the involved person
- The tools and methods of interaction, e.g. types and frequency of meetings, ground rules, conflict resolution, evaluation
- Desired patient / patient partner organisation to foster long-term working partnerships, with independence ensured (in scope)
- The profile of the type of patient/s or patient representatives/s to be involved and their number
- How activity outputs will be used and ownership of outputs
- How and when the patient/s involved will be informed of outcomes
- Contractual terms and conditions including consent and compensation (see section 11, written agreement).
- Other elements according to the specific project
Source
Download
Checklist
Patient identification
There are many ways to identify patients to be involved in an interaction. The main routes are through:
- Existing patient organizations
- EUPATI or similar project
- Advertising opportunities for patient participation
- Medical Key Opinion Leaders. healthcare providers, hospitals and researchers and other institutions
- Systematic social media search for patient exchange platforms
- Unsolicited requests previously made by interested parties
- Existing advisory boards / groups (e.g. EFPIA Think Tank, Patients and Consumers Working Party at the EMA)
- Specialized patient engagement agencies
Source: European Patients’Academy on Therapeutic Innovation (EUPATI) (2016): Guidance for patient involvement for industry-led medicines R&D. (12/06/17)
Download
Do's and Don'ts
in Collaboration with Patients and Patient Organizations
DO's
|
|
|
|
|
|
|
|
|
DON'Ts
|
|
|
|
|
|
|
|
|
|
|
|
Download

