EMA FDA HTA
Patient Engagement at EMA, FDA and HTA authorities
This chapter has been co-authored with Thomas Smith

Thomas Smith
is an international consultant providing expert patient insights. As a Cystic Fibrosis patient, a Member of the European Health Parliament and a Patient Expert to EURORDIS, he knows what matters for patients and how their interests can be made visible and powerfully advocated. Combining experience of policy, clinical research and media representation skills with an unshakeable belief that meaningful patient involvement is key for sustainability and meaningful innovation in healthcare, Thomas is a constructive challenger of the status quo.
Why Patient Engagement is important for Drug Approval and Reimbursement
Patient engagement or “involvement” in preparation of regulatory and health-technology-assessment (HTA) has become more and more important. In the following article, we provide an overview about the current status.
Patient Engagement becoming a key success factor at EMA and FDA
For years the European Medicines Agency(EMA) and the US Food and Drug Administration (FDA) have involved patients in their review processes. Patients and patient representatives in Europe and the US are constantly being searched for, educated and trained in large programs at EMA1 and the FDA2 to consult during these assessments. The understanding of the patient perspective today already has a strong impact on regulatory decision making. Watch the official videos below from both the European and the US-American agency about the importance of patient engagement in their review processes.
Hence since years both agencies have shown their commitment to involve patients early in the design of clinical trials and patient-friendly recruitment and information materials. They have urged drug developers consider systematic patient engagement on many occasions. However, to date patient involvement for industry has not been legally mandatory or directly requested by agency guidelines.
This may change: in June 2018 FDA released the Draft Guidance 1 of their Series of FDA Guidance for Enhancing the Incorporation of the Patient’s Voice in Drug Development and Regulatory Decision Making5. Shortly later they followed up with the discussion document on Patient Engagement in Medical Device Clinical Trials6.
How FDA paves the way
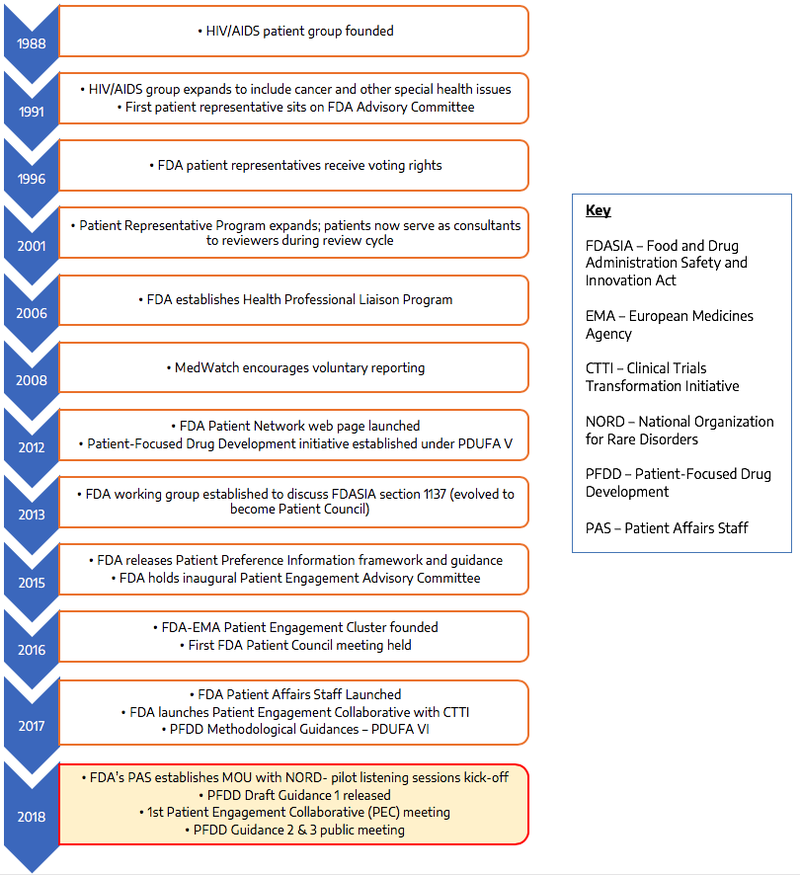
Source: Own presentation according to https://www.fda.gov/ForPatients/PatientEngagement/default.htm#evolution7
The EMAmay follow with similar guidance in the not too distant future as signaled in the regulatory science strategy “EMA regulatory science to 2025”8 Both agencies have joined forces in an effort to enhance patient engagement in their assessment processes by establishing the FDA and EMA Patient Engagement Cluster9.
Is Patient Engagement critical for HTA and favorable for pricing & reimbursement?
Also, in Health Technology Assessment the patient view on patient relevance of gathered evidence data (clinical and otherwise) is becoming of ever greater importance. Patient representatives are involved in assessments in more and more European countries and can move the needle with their contributions to the discussion or even their vote in the committees assigned to the matter. E.g. major European HTA authorities with strongly established patient involvement to date are NICE10 in the UK and GBA11 in Germany. In France significant efforts are ongoing to strengthen patient participation in the HTA processes of the French authority HAS12(the linked article also gives a good overview about the international status of HTA involvement).
As a concrete example, the demonstration of a patient-relevant benefit in the early assessment according to German law (§ 35b SGB V), is a prerequisite for later market access in Germany. The German Institute for Quality and Efficiency in Health Care assessing such patient benefit has defined patient relevance in 3.1.1 of its methodology guideline13. “In this connection, patient-relevant refers to how a patient feels, functions or survives”. In practice, the institute has considered direct feedback from patients about the impact of the intervention on their quality of life as extremely important to conclude on the quantification of the benefit of the new treatment. An analysis of the German GBA14 stressing the importance of such patient-relevance data shows the following results:
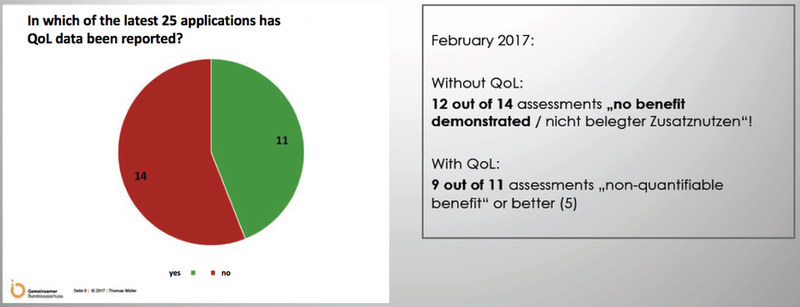
Source: https://www.vfa-patientenportal.de/download/praesentation-mueller13
This is an impressive example for how significant the impact of patient experience can be regarding health-related quality of life (HRQoL) withinHTA. However, all of these data had still been gathered from a very small group of patients in a randomized clinical trial (RCT).
How can we demonstrate true patient benefit other than through data from RCTs?
Certainly, the question of methodology and validation of quality of life (QOL) data / patient-reported outcome measures (PROs)/ real world data (RWD), within and of course outside of RCTs, is still a widely discussed subject. Seeking early guidance on what the EMA, FDA and HTA authorities will consider meaningful is essential. In Europe EUnetHTA and the EMA offer early dialogues and Parallel Consultations15 on evidence generation plans. But also national regulatory and HTA authorities across Europe offer early advice.
A first guideline of the EUnetHTA16 in collaboration with local agencies to the measurement of health-related-quality-of-life (HRQoL) for Relative Effectiveness Assessments underlines the effort of European HTA authorities to align their expectations. For the requirements of instruments to collect PROs they especially refer to the FDA (see Appendix 3). However, this guideline represents a consolidated view of non-binding recommendations only. In the end companies need to make sure to meet both, EMA and national HTA requirements.
Another promising project to support regulatory and HTA processes is theThe Patient Preferences in Benefit-Risk Assessments during the Drug Life Cycle (PREFER) project17under the EU Innovative Medicines Initiative. PREFER develops recommendations for guideline development of regulatory agencies, HTA bodies and industry concerning the involvement and collection of patient preference data in decision making.
Does it really move the needle?
But will collecting non-typical patient reported data from within an RCT really make a difference? Or -even more doubtful- will data from other sources outside of any regulated clinical trial environment, as e.g. data from patient registries or interviews and surveys -largely referred to by the vague term real world data (RWD) - be really hard enough to move the needle at the regulatory level? Well, here is a recent example we could contribute to. In 2018, EMA based their decision to maintain the orphan drug status for a drug for myotonic disorder mainly on RWD. They concluded the data showed sufficient evidence for patient harm due to lack of patient access to such drug treatment. Such evidence of harm was demonstrated through structured patient and physician interviews validated by a survey among 400 patients across 18 European countries!* This didn’t break the budget of the sponsor but contributed significantly to thousands of patients getting now access to the drug.
The European project Patients Active in Research and Dialogues for an Improved Generation of Medicines (PARADIGM), another sub-project of the EU Innovative MedicinesInitiative, is working on respective guidance and practical tools for patient organizations, regulatory and HTA agencies and industry. This large public-private partnership being co-led by the European Patients’ Forum18 (EPF) and the European Federation of Pharmaceutical Industries and Associations19 (EFPIA)is receiving significant funding from the European Union’s Horizon 202020 program and EFPIA. PARADIGM21 has a mission to resolve the following issue:
“For key stakeholders, such as researchers/drug developers, regulatory authorities, HTA bodies (reimbursement agencies), pertinent and basic issues across all groups are: who should be involved, how and when.” It is interesting to know that among PARADIGM’s key advisors there are also NICE, the UK HTA agency, the International Society For Pharmacoeconomics and Outcomes Research22 (ISPOR) and the International Consortium for Health Outcomes23 (ICHOM). All three are important players in shaping the future of value-based medicine. Results from Paradigm’s work can be found here: https://imi-paradigm.eu/petoolbox/
Get ready for meaningful patient engagement
Biotech, pharma and medtech should understand the strategic significance of these movements and initiatives and prepare accordingly. More than enough reasons for patient engagement already exist as demonstrated in another recent article of leading experts in the field.24
We highly recommend making good use of early dialogue and advice at EMA, EUNetHTA, and local agencies!
Philipp von Gallwitz and Christian Gross
admedicum®Business for Patients
* ”COMP concluded that "the sponsor’s structured survey which used a well-defined methodology together with the review done by COMP members demonstrated that there is limited and disrupted availability of mexiletine in the EU that results in patient harm, particularly in patients with non dystrophic myotonia. It is therefore established that Namuscla will bring significant benefit to those affected by the condition." (QUOTE from COMP assessment report page 16).
The assessment report can be found on the EMA website
Sources
Following we are listing helpful resources for further reading:
- DIA: Global Annual Meeting
- EMA: Training and resources for patients and consumers
- EMA: Video on Engaging with Patients
- FDA: CDER Regulatory Science: Patient-Focused Drug Development
- FDA: Patient-Focused Drug Development - Collecting Comprehensive and Representative Input
- FDA: Patient Engagement in Medical Device Clinical Trials
- FDA: Learn about FDA Patient Engagement
- FDA: For Patients
- EMA and FDA: Terms of reference on Patient Engagement
- NICE: Patient and Public Involvement Policy
- GBA: Patient Involvement
- GIENS Workshop: How to strenghten the presence of patients in HTA conducted by health authorities
- IQWiG: Methods paper on benefit assessment
- GBA (Germna): Early benefit assessment
- EMA EUnetHTA: Parallel consultation between EMA and EUnetHTA
- EUnetHTA: Health-related QoL and utility measures
- prefer: Patient preferences
- EUPATI: Europe for patients
- efpia: European Federation of Pharmaceutical Industries and Associations
- European Commission: Horizon 2020
- PARADIGM: Patients Active in Research and Dialogues for an Improved Generation of Medicines
- ISPOR: Society for health economics and outcomes research
- ICHOM: International consortium for health outcomes measurement

